The development of 2 resources to annotate and infer catheter-related infections in clinical adverse event incident reports.
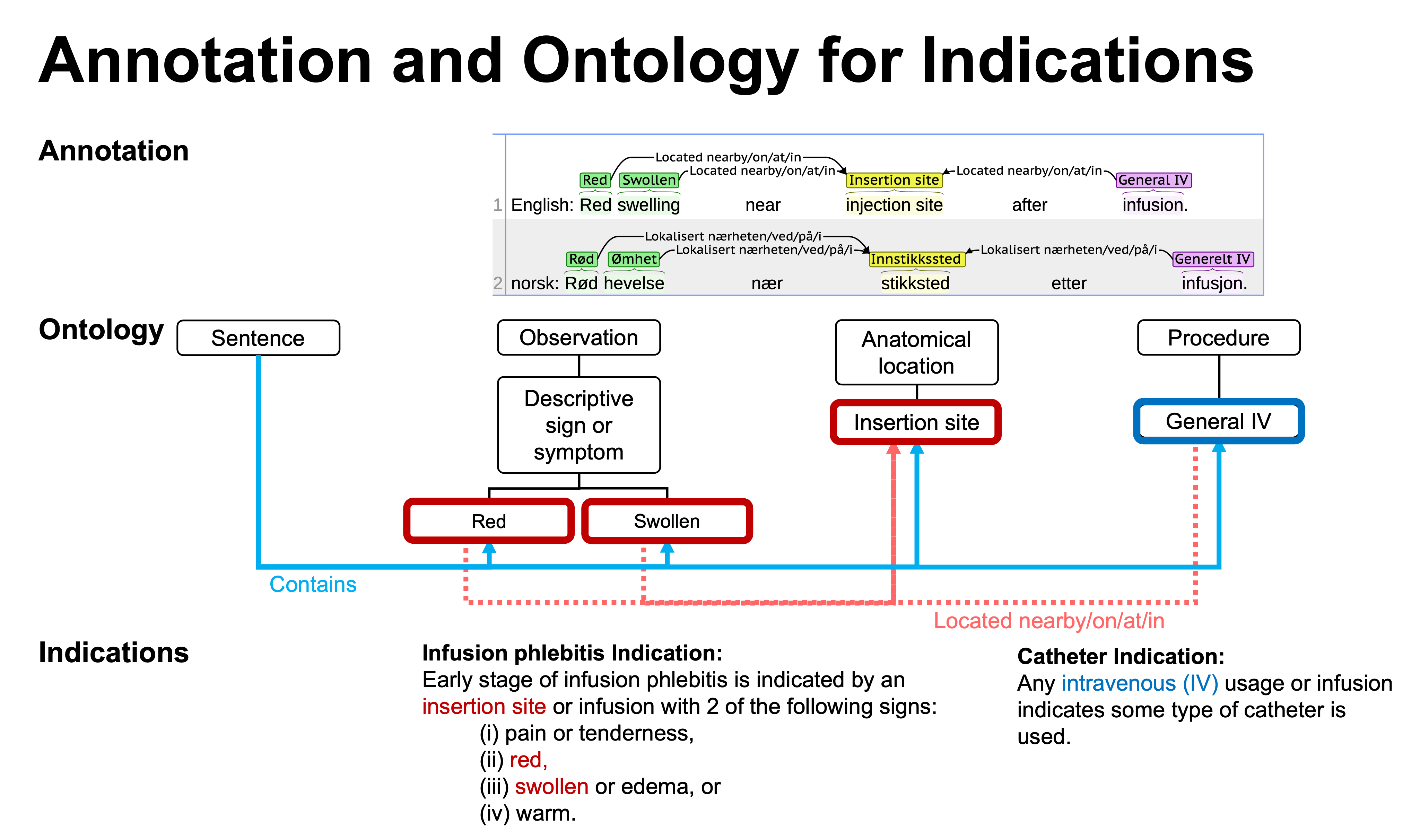
- Annotated Adverse Event NOte TErminology (AAENOTE) - the terminology used by annotators to annotate an adverse event document and it provides an index for annotated document.
- Catheter Infection Indications Ontology (CIIO) - the ontology complements AAENOTE by representing clinical knowledge needed to infer indications of catheters and infections.
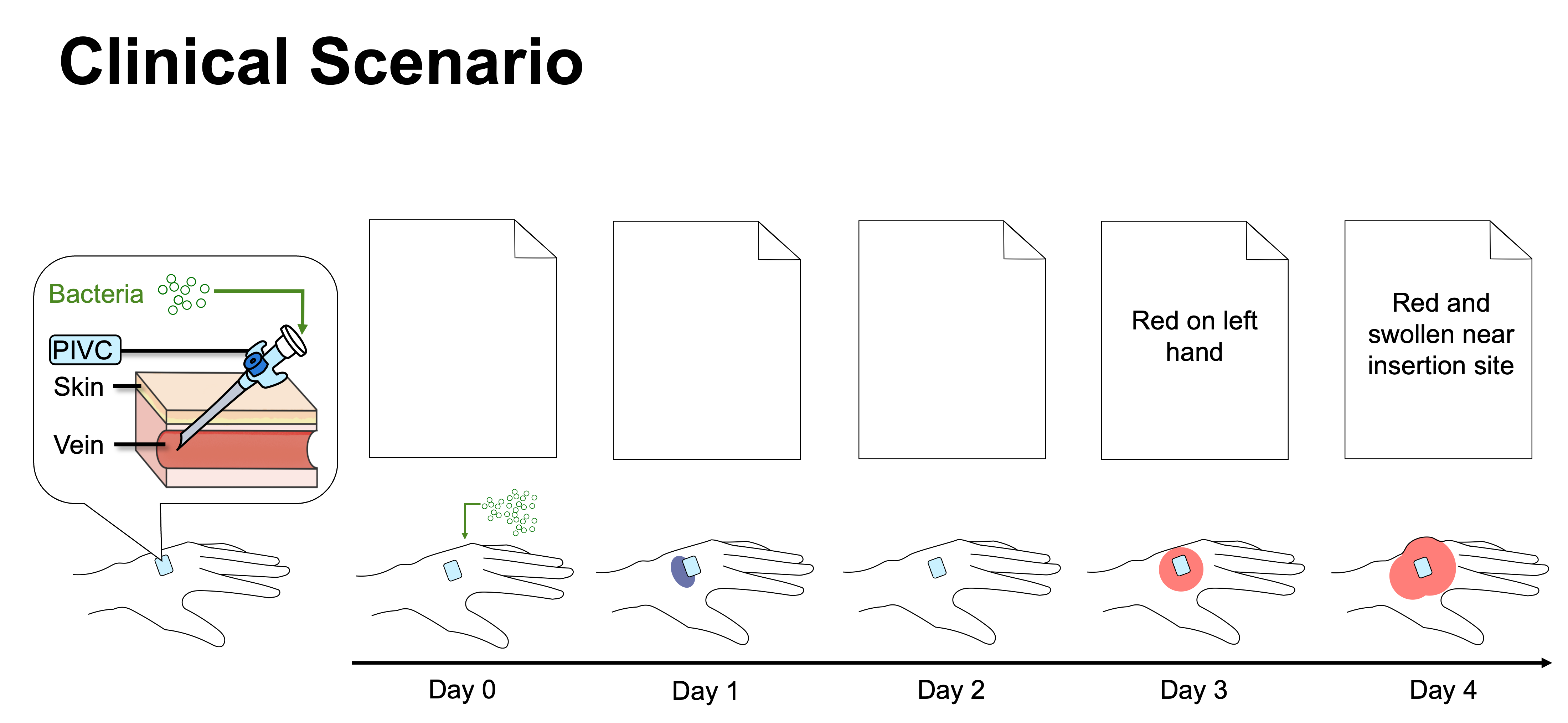
From a clinical scenario use case to annotated reports and an indication ontology to infer catheter-related infections. PIVC = peripheral intravenous catheter.
Goal: Create resources to systematically annotate text and reason about information in the annotations about catheter-related infections.
Method: Develop a terminology for annotators and an ontology for clinical reasoning with clinicians.
Use Case: In the clinical scenario, a hospitalized patient receives a peripheral intravenous catheter (PIVC) and bacteria enters. The early days show no documentation despite the catheter’s presence and bruising, while later visible inflammation is documented as “red” or “red and swollen near insertion site”. This illustrates a gap between actual events vs clinician documentation.
Results:
- A systematic semantic annotation method (Yan et al., 2023).
- An annotated corpus of incident reports for signs and events of catheters and infections (Yan et al., 2021).
- A clinician-verified terminology to reflect how catheter- and infection-related signs and events appear in clinical documentation (Yan et al., 2023).
- A corresponding clinician-verified ontology to infer the presence of documented catheters and infections (Yan et al., 2023).
GitHub: https://github.com/melissayan/aaenote_and_ciio
References
2023
-
Method for designing semantic annotation of sepsis signs in clinical text
Melissa Y Yan, Lise Tuset Gustad, Lise Husby Høvik, and Øystein Nytrø
In Proceedings of the 5th Clinical Natural Language Processing Workshop, 2023
Annotated clinical text corpora are essential for machine learning studies that model and predict care processes and disease progression. However, few studies describe the necessary experimental design of the annotation guideline and annotation phases. This makes replication, reuse, and adoption challenging. Using clinical questions about sepsis, we designed a semantic annotation guideline to capture sepsis signs from clinical text. The clinical questions aid guideline design, application, and evaluation. Our method incrementally evaluates each change in the guideline by testing the resulting annotated corpus using clinical questions. Additionally, our method uses inter-annotator agreement to judge the annotator compliance and quality of the guideline. We show that the method, combined with controlled design increments, is simple and allows the development and measurable improvement of a purpose-built semantic annotation guideline. We believe that our approach is useful for incremental design of semantic annotation guidelines in general.
-
Terminology and ontology development for semantic annotation: A use case on sepsis and adverse events
Melissa Y Yan, Lise Tuset Gustad, Lise Husby Høvik, and Øystein Nytrø
Semantic Web, May 2023
Annotations enrich text corpora and provide necessary labels for natural language processing studies. To reason and infer underlying implicit knowledge captured by labels, an ontology is needed to provide a semantically annotated corpus with structured domain knowledge. Utilizing a corpus of adverse event documents annotated for sepsis-related signs and symptoms as a use case, this paper details how a terminology and corresponding ontology were developed. The Annotated Adverse Event NOte TErminology (AAENOTE) represents annotated documents and assists annotators in annotating text. In contrast, the complementary Catheter Infection Indications Ontology (CIIO) is intended for clinician use and captures domain knowledge needed to reason and infer implicit information from data. The approach taken makes ontology development understandable and accessible to domain experts without formal ontology training.
2021
-
Preliminary Processing and Analysis of an Adverse Event Dataset for Detecting Sepsis-Related Events
Melissa Y Yan, Lise Husby Høvik, André Pedersen, Lise Tuset Gustad, and Øystein Nytrø
In IEEE International Conference on Bioinformatics and Biomedicine, BIBM 2021, Houston, TX, USA, December 9-12, 2021, May 2021
Adverse event (AE) reports contain notes detailing procedural and guideline deviations, and unwanted incidents that can bring harm to patients. Available datasets mainly focus on vigilance or post-market surveillance of adverse drug reactions or medical device failures. The lack of clinical-related AE datasets makes it challenging to study healthcare-related AEs. AEs affect 10% of hospitalized patients, and almost half are preventable. Having an AE dataset can assist in identifying possible patient safety interventions and performing quality surveillance to lower AE rates. The free-text notes can provide insight into the cause of incidents and lead to better patient care. The objective of this study is to introduce a Norwegian AE dataset and present preliminary processing and analysis for sepsis-related events, specifically peripheral intravenous catheter-related bloodstream infections. Therefore, the methods focus on performing a domain analysis to prepare and better understand the data through screening, generating synthetic free-text notes, and annotating notes.